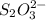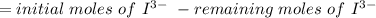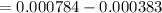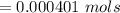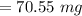Chemistry, 29.02.2020 02:54 kiarabermudez754
Calculate the weight percent of ascorbic acid in a tablet of Vitamin C from the following data:A 80 mg sample of a crushed Vitamin C tablet was dissolved in 40 mL of H2SO4 and 20 mL of water. Two grams of KI and 40. mL of 0.00653 M KIO3 solution was added, and the mixture titrated to a starch endpoint. The titration required 15 mL of 0.0510 M thiosulfate solution. I know the answer is 88%, but how did they get it?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, SmolBeanPotato
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 02:30, milesjreece3939
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 07:50, mckinleesmomp6qj1e
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 13:00, naomicervero
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3
You know the right answer?
Calculate the weight percent of ascorbic acid in a tablet of Vitamin C from the following data:A 80...
Questions in other subjects:

SAT, 09.12.2020 19:40

Mathematics, 09.12.2020 19:40



English, 09.12.2020 19:40

Chemistry, 09.12.2020 19:40

Mathematics, 09.12.2020 19:40


Mathematics, 09.12.2020 19:40

Mathematics, 09.12.2020 19:40



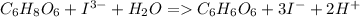
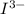 = 3 × number of moles of
= 3 × number of moles of 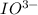
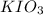
 mol
mol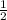 x moles of
x moles of