
Chemistry, 29.02.2020 01:51 heyysiirr3064
The first ionization potential of the elements B, C, and N (atomic numbers 5, 6, and 7) steadily increases, but that of O is less than that of N. The best interpretation of the lowervalue for O is that:1. the ionization potential of N is a maximum and the values decrease steadily for the elements O, F, and Ne.2. there is more shielding of the nuclear charge in O than in B, C, or N.3. the electron removed from O is farther from the nucleus and therefore less tightly bound than that in N.4. the electron removed from O corresponds to a different value of the quantum number L than that of the electron removed from B, C, or N.5. the half-filled set of p orbitals in N makes it more difficult to remove an electron from N than from O.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:30, rosetoheart2
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1
You know the right answer?
The first ionization potential of the elements B, C, and N (atomic numbers 5, 6, and 7) steadily inc...
Questions in other subjects:

Geography, 06.04.2020 01:02


Mathematics, 06.04.2020 01:02


History, 06.04.2020 01:02




Biology, 06.04.2020 01:02

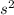 2
2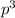 (half-filled orbital), Which allows it additional stability.
(half-filled orbital), Which allows it additional stability.


