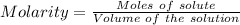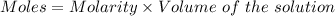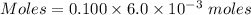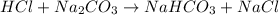Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 16:30, ccispoppin12
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
Calculate the number of moles of sodium carbonate originally present, if 6.0 ml of 0.100 M HCl were...
Questions in other subjects:


Mathematics, 10.02.2021 17:50


History, 10.02.2021 17:50

Chemistry, 10.02.2021 17:50

Mathematics, 10.02.2021 17:50

Chemistry, 10.02.2021 17:50

Mathematics, 10.02.2021 17:50

Mathematics, 10.02.2021 17:50