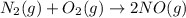The question is incomplete, complete question is;
For the reaction:
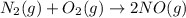
classify each of the following actions by whether it causes a leftward shift, a rightward shift, or no shift in the direction of the reaction.
a) half oxygen
b) double oxygen
c) double nitrogen
d) half nitrogen
e) double nitrogen monoxide
f) half nitrogen monoxide
Explanation:
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle.
This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
On an addition of product
If we add more product at an equilibrium, the equilibrium will move in backward direction towards the reactant side and vice versa.If we add more reactant at an equilibrium, the equilibrium will move in forward direction towards the product side and vice versa.
a) Half oxygen
Here reactant is reducing, which means that concentration of product is increasing at equilibrium. So, to in order to counter the change the equilibrium will move backward that is leftward direction .
b) Double oxygen
Here reactant is increasing , which means that concentration of product is deceasing at equilibrium. So, to in order to counter the change the equilibrium will move forward that is rightward direction.
c) Double nitrogen
Here reactant is increasing , which means that concentration of product is decreasing at equilibrium. So, to in order to counter the change the equilibrium will move forward that is rightward direction.
d) Half nitrogen
Here reactant is reducing, which means that concentration of product is increasing at equilibrium. So, to in order to counter the change the equilibrium will move backward that is leftward direction .
e) Double nitrogen monoxide
Here product is increasing , which means that concentration of reactant is decreasing at equilibrium. So, in order to counter the change the equilibrium will move backward that is leftward direction.
f) Half nitrogen monoxide
Here product is decreasing , which means that concentration of reactant is increasing at equilibrium. So, in order to counter the change the equilibrium will move forward that is rightward direction.