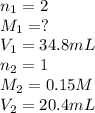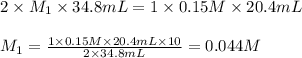Chemistry, 28.02.2020 23:18 smhrosepetals
If it takes 20.4 mL of NaOH(aq) to reach the equivalence point of the titration, what is the molarity of H2SO4(aq)? For your answer, only type in the numerical value with two significant figures. Do NOT include the unit.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, carsonjohnsonn
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 06:00, coopera1744
Find the mass in grams of 1.37x1020 particles of h3po4
Answers: 2

Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1
You know the right answer?
If it takes 20.4 mL of NaOH(aq) to reach the equivalence point of the titration, what is the molarit...
Questions in other subjects:








Mathematics, 04.04.2020 11:38


Advanced Placement (AP), 04.04.2020 11:39

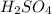 (aq) of an unknown concentration was titrated with 0.15 M of NaOH(aq).
(aq) of an unknown concentration was titrated with 0.15 M of NaOH(aq).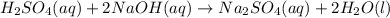
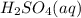 ? For your answer, only type in the numerical value with two significant figures. Do NOT include the unit.
? For your answer, only type in the numerical value with two significant figures. Do NOT include the unit.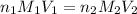
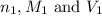 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 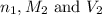 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.