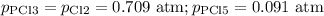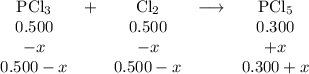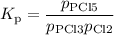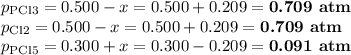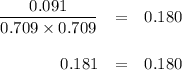Chemistry, 29.02.2020 00:17 Dogtes9667
For the exothermic reaction
PCl3(g)+Cl2(g)?PCl5(g)
Kp = 0.180 at a certain temperature.
A flask is charged with 0.500 atm PCl3 , 0.500 atm Cl2, and 0.300atm PCl5 at this temperature.
What are the equilibrium partial pressures of PCl3 , Cl2, and PCl5, respectively?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, giraffegurl
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 04:30, salvadorperez26
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2


You know the right answer?
For the exothermic reaction
PCl3(g)+Cl2(g)?PCl5(g)
Kp = 0.180 at a certain temperature.<...
PCl3(g)+Cl2(g)?PCl5(g)
Kp = 0.180 at a certain temperature.<...
Questions in other subjects:

Chemistry, 28.08.2019 21:30


Mathematics, 28.08.2019 21:30

Mathematics, 28.08.2019 21:30

English, 28.08.2019 21:30



Mathematics, 28.08.2019 21:30

History, 28.08.2019 21:30