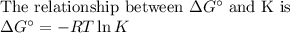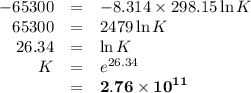Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, lindseyklewis1p56uvi
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1

Chemistry, 21.06.2019 19:30, umimgoingtofail
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

Chemistry, 22.06.2019 02:20, kristieroth1
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1
You know the right answer?
Sing any data you can find in the ALEKS Data resource, calculate the equilibrium constant K at 25.0°...
Questions in other subjects:



History, 03.02.2021 07:30


Business, 03.02.2021 07:30



Physics, 03.02.2021 07:30