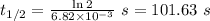Chemistry, 28.02.2020 19:45 brianadee800
The first-order rate constant for the decomposition of N2O5, 2N2O5(g)→4NO2(g)+O2(g) at 70∘C is 6.82×10−3 s−1. Starting with 8.00×10−2 mol of N2O5(g) in a volume of 2.9 L, how many moles of reactant are left after 5 minutes? What is its half-life?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kkruvc
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1


Chemistry, 22.06.2019 17:40, adantrujillo1234
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 23.06.2019 01:00, zitterkoph
Which of the following is a physical change? a. burning a piece of wood b. sawing a piece of wood in half c. rust forming on an iron fence d. a copper roof changing color from orange to green
Answers: 1
You know the right answer?
The first-order rate constant for the decomposition of N2O5, 2N2O5(g)→4NO2(g)+O2(g) at 70∘C is 6.82×...
Questions in other subjects:


Geography, 15.10.2019 05:00

Mathematics, 15.10.2019 05:00






Biology, 15.10.2019 05:00

![[A_t]=[A_0]e^{-kt}](/tpl/images/0528/2808/1ef89.png)
![[A_t]](/tpl/images/0528/2808/5262c.png) is the concentration at time t
is the concentration at time t ![[A_0]](/tpl/images/0528/2808/9a686.png) is the initial concentration =
is the initial concentration =  mol
mol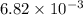 s⁻¹
s⁻¹![[A_t]=8.00\times 10^{-2}e^{-6.82\times 10^{-3}\times 300}\ mol=0.01034\ mol](/tpl/images/0528/2808/69780.png)