
Chemistry, 28.02.2020 19:21 semajac11135
The half-life of a first-order reaction is 13 min. If the initial concentration of reactant is 0.13 M, it takes min for it to decrease to 0.085 M. The half-life of a first-order reaction is 13 min. If the initial concentration of reactant is 0.13 M, it takes min for it to decrease to 0.085 M. 10. 11 7.0 12 8.0

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:50, scavalieri2421
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 05:30, smartie80
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 23.06.2019 01:00, carson9373
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

Chemistry, 23.06.2019 06:30, artbydi
Achemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 no(g) + cl2(g) < => 2 nocl(g) kp = 2 x 10^(-6)he fills a reaction vessel at this temperature with 13. atm of nitrogen monoxide gas and 12. atm of chlorine gas. use this data to answer the questions: a. can you predict the equilibrium pressure of noci, using only the tools available to you within aleks? y/nb. if you said yes, then enter the equilibrium pressure of nocl at right. round your answer to 1 significant digit.
Answers: 1
You know the right answer?
The half-life of a first-order reaction is 13 min. If the initial concentration of reactant is 0.13...
Questions in other subjects:

History, 11.10.2020 01:01


Mathematics, 11.10.2020 01:01

History, 11.10.2020 01:01

Mathematics, 11.10.2020 01:01




Mathematics, 11.10.2020 01:01

Mathematics, 11.10.2020 01:01

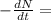 λN
λN t time t = 0.
t time t = 0.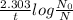

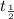 = 13 min
= 13 min
![\textrm{rate of reaction}=-\frac{d[A]}{dt} =k[A]](/tpl/images/0528/1630/bb97d.png)
![k=\frac{2.303}{t} log\frac{[A_0]}{[A]}](/tpl/images/0528/1630/263ab.png)

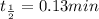


![\Rightarrow t=\frac{2.303}{k} log\frac{[A_0]}{[A]}](/tpl/images/0528/1630/3dc05.png)
 , [A₀] = 0.13 m and [ A] = 0.085 M
, [A₀] = 0.13 m and [ A] = 0.085 M



