
Chemistry, 28.02.2020 19:28 ballerboles4747
The radioisotope phosphorus-32 is used in tracers for measuring phosphorus uptake by plants. The half-life of phosphorus-32 is 14.3 days. If you begin with 30.5 mg of this isotope, what mass remains after 27.5 days have passed? Since the decomposition is a radioactive decay reaction, it is first order.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1



Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
You know the right answer?
The radioisotope phosphorus-32 is used in tracers for measuring phosphorus uptake by plants. The hal...
Questions in other subjects:

Mathematics, 11.09.2020 04:01

Mathematics, 11.09.2020 04:01

Mathematics, 11.09.2020 04:01

Mathematics, 11.09.2020 04:01

English, 11.09.2020 04:01

Mathematics, 11.09.2020 04:01

Mathematics, 11.09.2020 04:01

History, 11.09.2020 04:01

Mathematics, 11.09.2020 04:01

Mathematics, 11.09.2020 04:01

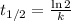
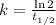
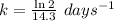
![[A_t]=[A_0]e^{-kt}](/tpl/images/0528/1963/1ef89.png)
![[A_t]](/tpl/images/0528/1963/5262c.png) is the concentration at time t
is the concentration at time t
![[A_0]](/tpl/images/0528/1963/9a686.png) is the initial concentration = 30.5 mg
is the initial concentration = 30.5 mg![[A_t]=30.5\times e^{-0.04847\times 27.5}\ mg=8.043\ mg](/tpl/images/0528/1963/500e7.png)


