
Chemistry, 28.02.2020 19:28 alexantkoviak13
The rate of disappearance of HBr in the gas phase reaction 2HBr(g)→H2(g)+Br2(g) is 0.360 Ms−1 at 150 ∘C. The rate of appearance of Br2 is Ms−1. The rate of disappearance of in the gas phase reaction is 0.360 at 150 . The rate of appearance of is . 1.39 0.600 0.720 0.180 0.130

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:00, levelebeasley1
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 23.06.2019 01:30, yarrito20011307
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 04:20, milkshakegrande101
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1
You know the right answer?
The rate of disappearance of HBr in the gas phase reaction 2HBr(g)→H2(g)+Br2(g) is 0.360 Ms−1 at 150...
Questions in other subjects:


Mathematics, 03.03.2021 17:20

Spanish, 03.03.2021 17:20

Computers and Technology, 03.03.2021 17:20



Geography, 03.03.2021 17:20

Geography, 03.03.2021 17:20


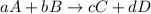
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0528/1964/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0528/1964/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0528/1964/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0528/1964/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0528/1964/d4b94.png)
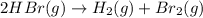
![\text{Rate of disappearance of }HBr=-\frac{1}{2}\frac{d[HBr]}{dt}](/tpl/images/0528/1964/d63dd.png)
![\text{Rate of appearance of }H_2=+\frac{d[H_2]}{dt}](/tpl/images/0528/1964/7fea8.png)
![\text{Rate of appearance of }Br_2=+\frac{d[Br_2]}{dt}](/tpl/images/0528/1964/d3d56.png)
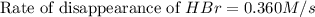
![+\frac{d[Br_2]}{dt}=-\frac{1}{2}\frac{d[HBr]}{dt}](/tpl/images/0528/1964/3e9c6.png)
![\frac{d[Br_2]}{dt}=\frac{1}{2}\times 0.360M/s](/tpl/images/0528/1964/53e73.png)
![\frac{d[Br_2]}{dt}=0.180M/s](/tpl/images/0528/1964/3fdb1.png)



