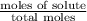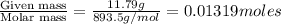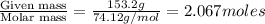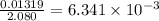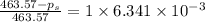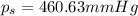Chemistry, 28.02.2020 19:30 samantha636
The vapor pressure of diethyl ether (ether) is 463.57 mm Hg at 25 °C. A nonvolatile, nonelectrolyte that dissolves in diethyl ether is chlorophyll. Calculate the vapor pressure of the solution at 25 °C when 11.79 grams of chlorophyll, C55H72MgN4O5 (893.5 g/mol), are dissolved in 153.2 grams of diethyl ether. diethyl ether = CH3CH2OCH2CH3 = 74.12 g/mol. VP(solution) = mm Hg

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:30, jade468
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1

Chemistry, 23.06.2019 00:00, kittenalexis68
How many atoms or molecules are there in a mole of a substance?
Answers: 1

Chemistry, 23.06.2019 01:30, Thunderalesis7855
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1

Chemistry, 23.06.2019 10:00, lexusdixon3
Which number should be placed before f2 on the reactants side equation to make equation balanced? xe + > xef4
Answers: 1
You know the right answer?
The vapor pressure of diethyl ether (ether) is 463.57 mm Hg at 25 °C. A nonvolatile, nonelectrolyte...
Questions in other subjects:


History, 31.08.2021 21:40





Mathematics, 31.08.2021 21:40

Chemistry, 31.08.2021 21:40


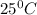 is 460.63 mmHg
is 460.63 mmHg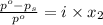
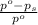 = relative lowering in vapor pressure
= relative lowering in vapor pressure
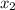 = mole fraction of solute =
= mole fraction of solute =