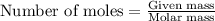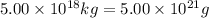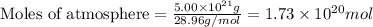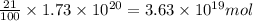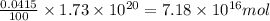The total mass of the atmosphere is about 5.00 x 1018 kg. How many moles each of air, O2, and CO2 are present in the atmosphere? Note that it is important to work in units of moles rather than in units of mass. By the ideal gas law, PV=nRT. P is pressure, V is volume, n is the number of moles, T is temperature (K), and R is the gas constant. At a given temperature and pressure, the volume is proportional to the number of moles, not to the mass.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, twinkieslayer
As a part of an experiment a student burns propane to produce carbon dioxide and water she remembers that she must follow the law conservation of matter when writing a balanced chemical equation which of these equation adheres to the law of conservation of matter
Answers: 1

Chemistry, 22.06.2019 16:00, sassy11111515
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 23.06.2019 03:40, ElegantEmerald
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
The total mass of the atmosphere is about 5.00 x 1018 kg. How many moles each of air, O2, and CO2 ar...
Questions in other subjects:


Mathematics, 13.04.2021 01:00



English, 13.04.2021 01:00



Mathematics, 13.04.2021 01:00

Mathematics, 13.04.2021 01:00

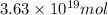 and
and 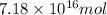 respectively
respectively