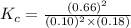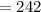Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, david838843
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 00:00, melidacampos12
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 10:50, lejeanjamespete1
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

You know the right answer?
What is the numerical value of KcKc for the reaction if the concentrations at equilibrium are 0.10 M...
Questions in other subjects:



History, 02.02.2020 21:46


Mathematics, 02.02.2020 21:46

Physics, 02.02.2020 21:46


Physics, 02.02.2020 21:46

Mathematics, 02.02.2020 21:46

Mathematics, 02.02.2020 21:46

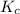 is 242
is 242![K_{c}=\frac{[SO_{3}]^{2}}{[SO_{2}]^{2}[O_{2}]}](/tpl/images/0528/1239/385cc.png)
![[SO_{3}]=0.66M](/tpl/images/0528/1239/eb431.png) ,
, ![[SO_{2}]=0.10M](/tpl/images/0528/1239/7c939.png) and
and ![[O_{2}]=0.18M](/tpl/images/0528/1239/a7908.png)