Chemistry, 28.02.2020 04:38 shelbylynn17
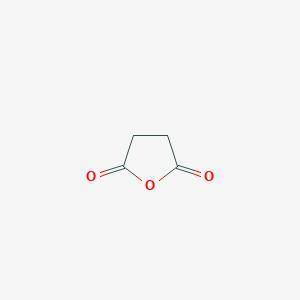
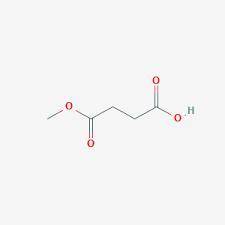
A compound A has prominent infrared absorptions at 1050, 1786, and 1852 cm–1 and shows a single absorption in the proton NMR spectrum at δ 3.00. When heated gently with methanol, compound B, C5H8O4, is obtained. Compound B has IR absorptions at 2500–3000 (broad), 1730, and 1701 cm–1, and its proton NMR spectrum in D2O consists of resonances at δ 2.7 (complex splitting) and δ 3.7 (a singlet) in the intensity ratio 4:3. Give the structures A and B, omitting stereochemistry.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:30, hdhshshs741
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1

Chemistry, 23.06.2019 05:30, zeesharpe05
If c + di is a point on the circle, then | c + di |=
Answers: 2

Chemistry, 23.06.2019 05:50, kawaunmartinjr10
Aseismic wave is energy released as the result of rock movement along a fault. t or f ?
Answers: 1
You know the right answer?
A compound A has prominent infrared absorptions at 1050, 1786, and 1852 cm–1 and shows a single abso...
Questions in other subjects:


Social Studies, 12.03.2022 01:00



English, 12.03.2022 01:00

Computers and Technology, 12.03.2022 01:00