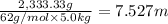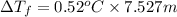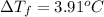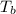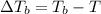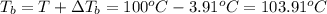Some ethylene glycol, , is added to your car’s cooling system along with 5.0 kg of water.
a. If the freezing point of the water–glycol solution is −14.0 °C, what mass of must have been added?
b. What is the boiling point of the coolant mixture? Kb(H20) = 0.52 degrees celcius kg mol^-1.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, veronica25681
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
Some ethylene glycol, , is added to your car’s cooling system along with 5.0 kg of water.
Questions in other subjects:


Mathematics, 25.01.2020 03:31



Mathematics, 25.01.2020 03:31





Health, 25.01.2020 03:31

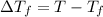
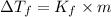
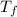 = freezing point of solution
= freezing point of solution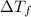 =depression in freezing point
=depression in freezing point 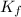 = freezing point constant
= freezing point constant 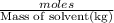
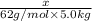
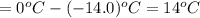
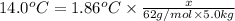
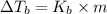
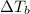 =elevation in boiling point =
=elevation in boiling point = 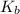 = boiling point constant
= boiling point constant