
Chemistry, 28.02.2020 04:02 TrapQueen665
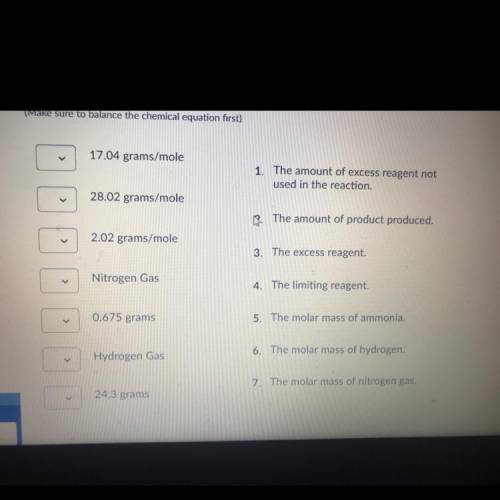
The haber process involves nitrogen gas combining with hydrogen gas to produce ammonia. If 20.0 grams of nitrogen gas combines with 5.0 grams of hydrogen gas, find the following: the molar mass of reactants and products, limiting reactant, the excess reactant, the amount of ammonia produced, the amount of excess chemical not used in the reaction.
Nitrogen gas+ hydrogen gas <——> ammonia gas
N2 + H2 -> NH3
(Make sure to balance the chemical equation first)
A lot of points !!!

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, myamiller558
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1


Chemistry, 22.06.2019 13:00, yaneiryx5476
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1
You know the right answer?
The haber process involves nitrogen gas combining with hydrogen gas to produce ammonia. If 20.0 gram...
Questions in other subjects:

History, 01.12.2019 01:31


Mathematics, 01.12.2019 01:31

Physics, 01.12.2019 01:31

Mathematics, 01.12.2019 01:31





Mathematics, 01.12.2019 01:31



