
Chemistry, 27.02.2020 22:19 lizzytyler4725
A 10.000g ice cube is added to 100.000g of water at 80.0 oC in a calorimeter. The final temperature of the water in the calorimeter is 65.5 oC. How much heat in kilojoules was needed to melt the ice cube?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 17:40, adantrujillo1234
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 21:50, SoccerAllStar2
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 23.06.2019 00:30, mariaramirez110379
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
A 10.000g ice cube is added to 100.000g of water at 80.0 oC in a calorimeter. The final temperature...
Questions in other subjects:



Computers and Technology, 18.09.2019 16:30

Biology, 18.09.2019 16:30


Mathematics, 18.09.2019 16:30

Physics, 18.09.2019 16:30



Chemistry, 18.09.2019 16:30



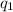 = amount of heat absorbed = ?
= amount of heat absorbed = ? = enthalpy change for fusion = 334.16 J/g
= enthalpy change for fusion = 334.16 J/g

 = heat absorbed
= heat absorbed = change in temperature =
= change in temperature = ![T_2-T_1=[65.0-0]^oC=65.5^oC](/tpl/images/0527/2479/63e8e.png)


![[3341.6+2741.83]J=6083.43J=6.083kJ](/tpl/images/0527/2479/8ebc5.png)


