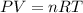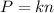Two flasks of equal volume and at the same temperature contain different gases. One flask contains 10.0 g of Ne, and the other flask contains 10.0 g of He. Is each of the following statements true or false? Explain.1) True. Because the gases have the same mass, they exert the same pressures.2) True. Both the volumes and the temperatures of the gases are the same, which means that their pressures must be equal.3) False. There are different numbers of moles in the flasks, meaning that the pressures are different.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, jeffcarpenter
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 09:30, lisbet123085
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1
You know the right answer?
Two flasks of equal volume and at the same temperature contain different gases. One flask contains 1...
Questions in other subjects:

Mathematics, 23.04.2021 17:20

Mathematics, 23.04.2021 17:20

Biology, 23.04.2021 17:20





Mathematics, 23.04.2021 17:20

English, 23.04.2021 17:20