
Chemistry, 27.02.2020 18:30 pineapplefun
Immunoglobulin G(IgG), formerly called gamma globulin, is a principle antibody in blood serum. A 0.457 gram sample of immunoglobulin G is dissolved in water to make 0.123 L of solution, and the osmotic pressure of the solution at 25C is found to be 0.614 mbar. Calculate the molecular mass of immunoglobulin G.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:00, annsmith66
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 03:40, 19thomasar
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 06:20, stephliu721
What is a property of a double replacement reaction
Answers: 1
You know the right answer?
Immunoglobulin G(IgG), formerly called gamma globulin, is a principle antibody in blood serum. A 0.4...
Questions in other subjects:

SAT, 07.01.2022 17:00



English, 07.01.2022 17:00


SAT, 07.01.2022 17:10




Biology, 07.01.2022 17:10

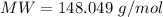 where MW is molar mass
where MW is molar mass


