
A chemistry graduate student is studying the rate of this reaction: 2H3PO4 (aq) ---> P2O5 (aq) + 3H2O (aq)He fills a reaction vessel with and measures its concentration as the reaction proceeds: time (seconds) [H3PO4]0 0.03M1 0.018M2 0.011M3 0.0067M4 0.0041MUse this data to answer the following questions. Write the rate law of the reaction.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, itzhari101
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 20:30, ShahinF7536
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1

You know the right answer?
A chemistry graduate student is studying the rate of this reaction: 2H3PO4 (aq) ---> P2O5 (aq) +...
Questions in other subjects:




History, 27.07.2019 22:00




Mathematics, 27.07.2019 22:00


Computers and Technology, 27.07.2019 22:00

![R=k[H_3PO_4]^1](/tpl/images/0526/7836/e1aa5.png)
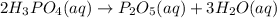
![R=k[H_3PO_4]^a](/tpl/images/0526/7836/abe08.png)
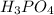 and k is rate constant.
and k is rate constant.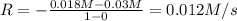
![0.012 M/s=k[0.018 M]^a](/tpl/images/0526/7836/4e403.png) ..[1]
..[1]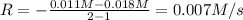
![0.007 M/s=k[0.011 M]^a](/tpl/images/0526/7836/deca4.png) ..[2]
..[2]![\frac{0.012 M/s}{0.007M/s}=\frac{k[0.018 M]^a}{k[0.011 M]^a}](/tpl/images/0526/7836/891ed.png)


