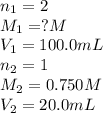Chemistry, 27.02.2020 05:47 utjfkdndidndldn62121
A sample of oxalic acid (a diprotic acid of the formula H2C2O4) is dissolved in enough water to make 1.00 L of solution. A 100.0 mL sample of this solution is titrated with a solution of sodium hydroxide of concentration 0.750 M and requires 20.0 mL of sodium hydroxide to reach the end point. Calculate the mass of the original oxalic acid sample.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 13:30, michealsfamily
The activation energy for a(n) is quite large and usually takes extra energy from the environment, it is normally not a natural spontaneous process. combustion reaction endothermic reaction exothermic reaction catalyzed reaction
Answers: 1

Chemistry, 23.06.2019 16:30, ernie27
In chile, the deepest earthquake occurred at 61.7°w longitude at a depth of 540 km. if the rocks at the focus began subducting 10 million years ago and are now 1000 km from their original position, what is the average rate of subduction in cm/yr?
Answers: 2
You know the right answer?
A sample of oxalic acid (a diprotic acid of the formula H2C2O4) is dissolved in enough water to make...
Questions in other subjects:

Mathematics, 12.04.2021 20:00

Mathematics, 12.04.2021 20:00



Mathematics, 12.04.2021 20:00

Social Studies, 12.04.2021 20:00


Mathematics, 12.04.2021 20:00


Business, 12.04.2021 20:00

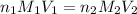
 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 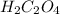
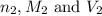 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.