
Chemistry, 27.02.2020 04:55 wileycheyennem
What is the solubility of calcium sulfate (CaSO4) in 0.30 M aqueous sodium sulfate (Na2SO4)? (Ksp of calcium sulfate = 2.0 x 10^-5

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:20, Richwave17
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 14:30, cxttiemsp021
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
What is the solubility of calcium sulfate (CaSO4) in 0.30 M aqueous sodium sulfate (Na2SO4)? (Ksp of...
Questions in other subjects:


Mathematics, 05.07.2019 16:30

Physics, 05.07.2019 16:30

Health, 05.07.2019 16:30

Mathematics, 05.07.2019 16:30


Mathematics, 05.07.2019 16:30

History, 05.07.2019 16:30

History, 05.07.2019 16:30

![K_{sp}=[Ca^{2+}][SO_4^{2-}]](/tpl/images/0526/4512/958f2.png) ........(1)
........(1)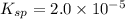
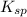 and [SO₄²⁻] in equation (1)
and [SO₄²⁻] in equation (1)![2.0\times 10^{-5}= [Ca^{2+}]0.30](/tpl/images/0526/4512/53779.png)
![\Rightarrow [Ca^{2+}]=\frac{2.0\times 10^{-5}}{0.30}](/tpl/images/0526/4512/70356.png)


