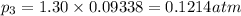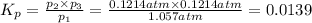Chemistry, 27.02.2020 02:54 MacieKay8865
At 25°C, gaseous decomposes to and to the extent that 10.3% of the original (by moles) has decomposed to reach equilibrium. The total pressure (at equilibrium) is 1.30 atm. Calculate the value of for this system. =

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:10, angellong94
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 23.06.2019 00:30, DragonLovely
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 07:30, bryantjorell
Which statement is actually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 2
You know the right answer?
At 25°C, gaseous decomposes to and to the extent that 10.3% of the original (by moles) has decompose...
Questions in other subjects:

Biology, 28.07.2019 07:00

Social Studies, 28.07.2019 07:00

History, 28.07.2019 07:00




Social Studies, 28.07.2019 07:00

History, 28.07.2019 07:00

Biology, 28.07.2019 07:00

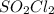 decomposes to
decomposes to 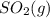 and
and 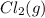 to the extent that 10.3% of the original
to the extent that 10.3% of the original 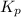 of the reaction is 0.0139.
of the reaction is 0.0139.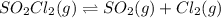
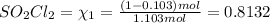
 (Dalton's law of partial pressure)
(Dalton's law of partial pressure)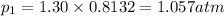
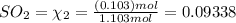
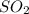 equilibrium ;
equilibrium ;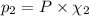 (Dalton's law of partial pressure)
(Dalton's law of partial pressure)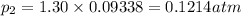
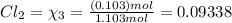
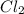 equilibrium ;
equilibrium ;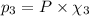 (Dalton's law of partial pressure)
(Dalton's law of partial pressure)