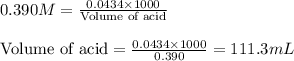Chemistry, 27.02.2020 02:21 jaidalynkimora
Suppose that you have 115 mL of a buffer that is 0.460 M in both benzoic acid ( C 6 H 5 COOH ) and its conjugate base ( C 6 H 5 COO − ) . Calculate the maximum volume of 0.390 M HCl that can be added to the buffer before its buffering capacity is lost.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, carlinryan
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

You know the right answer?
Suppose that you have 115 mL of a buffer that is 0.460 M in both benzoic acid ( C 6 H 5 COOH ) and i...
Questions in other subjects:


Biology, 24.03.2020 15:32

Mathematics, 24.03.2020 15:32

English, 24.03.2020 15:32


Mathematics, 24.03.2020 15:34

English, 24.03.2020 15:34

Mathematics, 24.03.2020 15:35

English, 24.03.2020 15:35

![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0526/1364/e4eea.png)
![pH=pK_a+\log(\frac{[C_6H_5COO^-]}{[C_6H_5COOH]})](/tpl/images/0526/1364/e41e3.png) .....(1)
.....(1)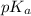 = negative logarithm of acid dissociation constant of benzoic acid = 4.2
= negative logarithm of acid dissociation constant of benzoic acid = 4.2![[C_6H_5COOH]=0.460M](/tpl/images/0526/1364/fc044.png)
![[C_6H_5COO^-]=0.460M](/tpl/images/0526/1364/ef922.png)
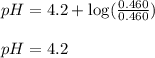
![3.2=4.2+\log(\frac{[C_6H_5COO^-]}{[C_6H_5COOH]})\\\\\frac{[C_6H_5COO^-]}{[C_6H_5COOH]}=0.1](/tpl/images/0526/1364/89428.png)
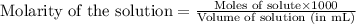 ......(2)
......(2)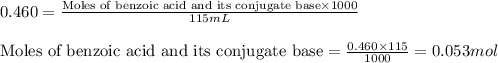
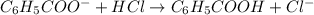
![\frac{[C_6H_5COO^-]-x}{[C_6H_5COOH]+x}=0.1\\\\\frac{0.053-x}{0.053+x}=0.1\\\\x=0.0434](/tpl/images/0526/1364/881c9.png)