
Chemistry, 27.02.2020 02:02 TombRaider167
Deep-sea divers must use special gas mixtures in their tanks, rather than compressed air, to avoid serious problems. One such breathing mixture contains helium, oxygen, and carbon dioxide. Determine the partial pressure of oxygen when the total pressure in the tank is 201.4 kPa if PHe = 125.4 kPa and PCO2= 18.2 kPa? Must show all work that leads to answer for credit

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, hunterthompson2
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2

Chemistry, 21.06.2019 22:30, ayoismeisalex
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 16:00, corcoranrobert1959
How do dying stars contribute to the formation of planets
Answers: 1
You know the right answer?
Deep-sea divers must use special gas mixtures in their tanks, rather than compressed air, to avoid s...
Questions in other subjects:

History, 30.10.2020 06:20

Mathematics, 30.10.2020 06:20





Arts, 30.10.2020 06:20



Arts, 30.10.2020 06:20

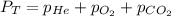
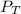 = 201.4 kPa
= 201.4 kPa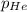 = 125.4 kPa
= 125.4 kPa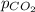 = 18.2 kPa
= 18.2 kPa![201.4=125.4+p_{O_2}+18.2\\\\p_{O_2}=201.4-[125.4+18.2]=57.8kPa](/tpl/images/0526/1098/fa869.png)


