
Chemistry, 27.02.2020 00:31 paytonrules3634
Reduction of iodate, IO3-(aq) + 5 H+(aq) + 4 e− → HIO(aq) + 2 H2O(aq), occurs with E0 = +1.13 V. What is the electrode potential, E, of the half reaction if the concentration/activity of all substances is 1.00 M?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, CoolRahim9090
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 23.06.2019 00:50, maddysmall32
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1


Chemistry, 23.06.2019 09:00, alisonlebron15
Are the results of a thoroughly tested hypothesis?
Answers: 2
You know the right answer?
Reduction of iodate, IO3-(aq) + 5 H+(aq) + 4 e− → HIO(aq) + 2 H2O(aq), occurs with E0 = +1.13 V. Wha...
Questions in other subjects:


Mathematics, 20.11.2020 18:20

English, 20.11.2020 18:20

Health, 20.11.2020 18:20

Mathematics, 20.11.2020 18:20

History, 20.11.2020 18:20

Mathematics, 20.11.2020 18:20

Mathematics, 20.11.2020 18:20

English, 20.11.2020 18:20

Mathematics, 20.11.2020 18:20

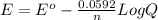
![E = E^o - \frac{0.0592}{n}LogQ\\\\E = 1.13 - \frac{0.0592}{4}Log[1]\\\\E =1.13 -0\\\\E =+1.13 V](/tpl/images/0525/9156/3694d.png)


