
Chemistry, 27.02.2020 00:10 barnhill6534
H2S(aq)⇌HS−(aq)+H+(aq), K1 = 9.06×10−8, and HS−(aq)⇌S2−(aq)+H+(aq), K2 = 1.18×10−19, what is the equilibrium constant Kfinal for the following reaction? S2−(aq)+2H+(aq)⇌H2S(aq)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 19:30, toriabrocks
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2
You know the right answer?
H2S(aq)⇌HS−(aq)+H+(aq), K1 = 9.06×10−8, and HS−(aq)⇌S2−(aq)+H+(aq), K2 = 1.18×10−19, what is the equ...
Questions in other subjects:

Mathematics, 28.05.2021 22:10




Social Studies, 28.05.2021 22:10


Mathematics, 28.05.2021 22:10

Mathematics, 28.05.2021 22:10


 is 9.35× 10²⁵
is 9.35× 10²⁵![k=\frac{[C]^z}{[A]^x[B]^y}](/tpl/images/0525/8601/0a5aa.png)

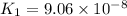
![K_1=\frac{[HS^-][H^+]}{[H_2S]}](/tpl/images/0525/8601/e1e1d.png)


![K_2=\frac{[S^{2-}][H^+]}{[HS^-]}](/tpl/images/0525/8601/661de.png)

![K_3=\frac{[H_2S]}{[S^{2-}][H^+]^2}](/tpl/images/0525/8601/58537.png)
![k_1k_2=\frac{[HS^-][H^+]}{[H_2S]}\frac{[S^{2-}][H^+]}{[HS^-]}](/tpl/images/0525/8601/53894.png)
![\Rightarrow k_1k_2=\frac{[S^{2-}][H^+]^2}{[H_2S]}](/tpl/images/0525/8601/762f9.png)







