
Chemistry, 26.02.2020 22:32 crzyemo865
For the chemical reaction below, determine the amount of HI produced when 3.32E+0 g of hydrogen is reacted with 5.064E+1 g of iodine to produce hydrogen iodide (HI). H(g) + I(g) → 2HI(g)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, debordc17
Two friends at different locations want to communicate with each other by sending low energy signals. which of the following methods can they use to communicate? a) produce x-rays using colliding electrons and send them to radios, which capture sound b) send messages using infrared radiation, which travel in the form of waves c) send radio waves through intervening media like radio and television d) produce sound waves using microwaves from heated objects
Answers: 2

Chemistry, 22.06.2019 14:30, KennyOaks6230
Which of the following units is not an official si unit
Answers: 1

Chemistry, 22.06.2019 14:50, jonmorton159
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

You know the right answer?
For the chemical reaction below, determine the amount of HI produced when 3.32E+0 g of hydrogen is r...
Questions in other subjects:


Biology, 28.09.2019 15:30


Biology, 28.09.2019 15:30

Mathematics, 28.09.2019 15:30


Social Studies, 28.09.2019 15:30



 .....(1)
.....(1)





 of hydrogen
of hydrogen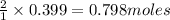 of hydrogen iodide
of hydrogen iodide 



