
Chemistry, 26.02.2020 21:32 ddmoorehouseov75lc
At equilibrium, the concentrations are [H2] = 5.0 M, [N2] = 10 M, and [NH3] = 3.0 M. What were the concentrations of nitrogen gas and hydrogen gas that were reacted initially?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, mercymain1014
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1


Chemistry, 22.06.2019 19:00, QuestionsAnsweredNow
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

Chemistry, 23.06.2019 01:30, heavendl13
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1
You know the right answer?
At equilibrium, the concentrations are [H2] = 5.0 M, [N2] = 10 M, and [NH3] = 3.0 M. What were the c...
Questions in other subjects:


Mathematics, 04.06.2020 13:57


Mathematics, 04.06.2020 13:57


Chemistry, 04.06.2020 13:57

Mathematics, 04.06.2020 13:57

Mathematics, 04.06.2020 13:57

Chemistry, 04.06.2020 13:57

![[H_2]_0=0.5M](/tpl/images/0525/5015/df212.png)
![[N_2]_0=8.5M](/tpl/images/0525/5015/80658.png)
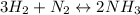
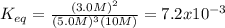
![\ \ \ \ \ \ \ \ \ \ \ \ 3H_2\ \ \ +\ \ \ \ N_2\ \ \ \ \leftrightarrow \ \ \ \ 2NH_3\\I\ \ \ \ \ \ \ \ \ \ \ [H_2]_0\ \ \ \ \ \ \ [N_2]_0\ \ \ \ \ \ \ \ \ \ \ \ \ \ 0\\C\ \ \ \ \ \ \ \ \ \ \ -3x\ \ \ \ \ \ \ \ \ \ x\ \ \ \ \ \ \ \ \ \ \ \ \ \ \ 2x\\E\ \ \ \ \ \ \ \ [H_2]_0-3x\ \ [N_2]_0-x\ \ \ \ \ \ \ \ \ 2x](/tpl/images/0525/5015/9f9db.png)
 " due to the reaction is computed via the equilibrium concentration of ammonia as shown below:
" due to the reaction is computed via the equilibrium concentration of ammonia as shown below: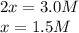
![[H_2]_0=5.0M-3(1.5M)=0.5M](/tpl/images/0525/5015/60025.png)
![[N_2]_0=10.0M-1.5M=8.5M](/tpl/images/0525/5015/9d364.png)


