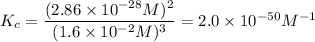Consider the reaction.
3 upper O subscript 2 (g) double-headed arrow 2 upper O subscript...

Chemistry, 26.02.2020 20:55 jessicajamah3289
Consider the reaction.
3 upper O subscript 2 (g) double-headed arrow 2 upper O subscript 3 (g).
At 298 K, the equilibrium concentration of O2 is 1.6 x 10-2 M, and the equilibrium concentration of O3 is 2.86 x 10-28 M. What is the equilibrium constant of the reaction at this temperature?
A) 2.0 x 10^-10
B) 2.0 x 10^10
C) 1.8 x 10^-10
D) 1.8 x 10^10

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:50, kukisbae
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 03:10, lilque6112
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 10:10, veronica022
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 10.06.2021 18:50


History, 10.06.2021 18:50



English, 10.06.2021 18:50

Mathematics, 10.06.2021 18:50

Mathematics, 10.06.2021 18:50

Mathematics, 10.06.2021 18:50


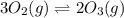
![K_c=\dfrac{[O_3g)]^2}{[O_2(g)]^3}](/tpl/images/0525/4129/a4203.png)