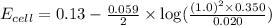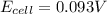Chemistry, 26.02.2020 20:54 adazeb2003
Calculate Ecell for the following electrochemical cell at 25 ºCPt (s) | H2 (g, 1.00 atm) | H+ (aq, 1.00 M) || Sn2+ (aq, 0.350 M) | Sn4+ (aq, 0.020 M) | Pt (s)The standard reduction potentials are as follows:Sn4+ (aq) + 2 e–à Sn2+ (aq) Eº = +0.13 V2 H+ (aq) + 2 e–à H2 (g) Eº = 0.00 V

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mayamabjishovrvq9
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2


You know the right answer?
Calculate Ecell for the following electrochemical cell at 25 ºCPt (s) | H2 (g, 1.00 atm) | H+ (aq, 1...
Questions in other subjects:


Chemistry, 11.10.2020 14:01



English, 11.10.2020 14:01



History, 11.10.2020 14:01

English, 11.10.2020 14:01


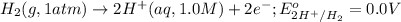
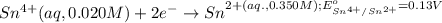
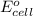 of the reaction, we use the equation:
of the reaction, we use the equation: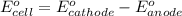

![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[H^{+}]^2[Sn^{2+}]}{[Sn^{4+}]}](/tpl/images/0525/4106/69569.png)
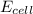 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V![[H^{+}]=1.00M](/tpl/images/0525/4106/641ea.png)
![[Sn^{2+}]=0.350M](/tpl/images/0525/4106/7d5cb.png)
![[Sn^{4+}]=0.020M](/tpl/images/0525/4106/c69f3.png)