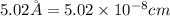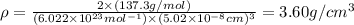Chemistry, 26.02.2020 19:16 hailiemanuel3461
Barium crystallizes in a body-centered cubic system with atoms at all lattice points and an edge length of 5.02 angstroms. Calculate its density in g/cm3.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

You know the right answer?
Barium crystallizes in a body-centered cubic system with atoms at all lattice points and an edge len...
Questions in other subjects:

History, 06.02.2021 06:30

Mathematics, 06.02.2021 06:30

Mathematics, 06.02.2021 06:30



History, 06.02.2021 06:30




Mathematics, 06.02.2021 06:30


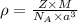 .............(1)
.............(1) = density = ?
= density = ?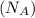 = Avogadro's number =
= Avogadro's number =