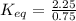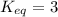Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, sgslayerkingminecraf
Which of the following statements about acidic water is true? a. acid has no effect on the h, o molecules. b. the solution contains a larger number of oh ions than h, o ions. c. the solution contains a larger number of h, o ions than qh ions. d. the solution contains an equal number of h, o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 23.06.2019 10:20, NasirKA7372
What do many bases have in common? apex chemistry sem 2
Answers: 2

Chemistry, 23.06.2019 13:20, TayJoker966
What volume of 24% trichloroacetic acid (tca) is needed to prepare eight 3 ounce bottles of 10% tca solution?
Answers: 2
You know the right answer?
In a reversible chemical reaction at equilibrium, the concentration of X (the reactant) is 0.75 mol/...
Questions in other subjects:

Mathematics, 09.06.2021 02:50

Health, 09.06.2021 02:50

English, 09.06.2021 02:50

Mathematics, 09.06.2021 02:50

Mathematics, 09.06.2021 02:50

Mathematics, 09.06.2021 02:50

Mathematics, 09.06.2021 02:50

Chemistry, 09.06.2021 02:50


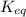 is, 3
is, 3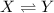
![K_{eq}=\frac{[Y]}{[X]}](/tpl/images/0525/1559/3a9b9.png)