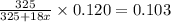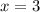Chemistry, 26.02.2020 18:29 unknown337
4. A 120.0 mg sample of a lead acetate hydrate, Pb(C2H3O2)2 × xH2O was heated to drive off the waters of hydration. The cooled residue had a mass of 103.0 mg. Calculate the value of x in the chemical formula. Show your work with Equation Editor.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, ian2006huang
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3


Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
4. A 120.0 mg sample of a lead acetate hydrate, Pb(C2H3O2)2 × xH2O was heated to drive off the wate...
Questions in other subjects:



Mathematics, 28.10.2020 22:00

Mathematics, 28.10.2020 22:00

Arts, 28.10.2020 22:00

Mathematics, 28.10.2020 22:00


SAT, 28.10.2020 22:00

Chemistry, 28.10.2020 22:00

Mathematics, 28.10.2020 22:00

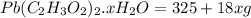
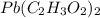 = 325 g
= 325 g
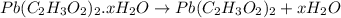
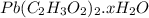 on heating gives = 325 g of
on heating gives = 325 g of 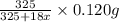 of
of