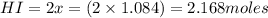Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:00, nyasiasaunders1234
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 09:30, psychocatgirl1
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone, due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
Calculate the number of moles of HI that are at equilibrium with 1.39 mol of H2 and 1.39 mol of I2 i...
Questions in other subjects:









Biology, 07.04.2020 19:30

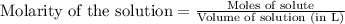

 for above equation follows:
for above equation follows:![K_c=\frac{[HI]^2}{[I_2][H_2]}](/tpl/images/0525/0428/8acbe.png)

![[HI]_{eq}=\frac{2x}{5.00}](/tpl/images/0525/0428/ab69d.png)
![[H_2]_{eq}=\frac{(1.39-x)}{5.00}](/tpl/images/0525/0428/598c6.png)
![[I_2]_{eq}=\frac{(1.39-x)}{5.00}](/tpl/images/0525/0428/0f5f3.png)