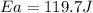Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, ulilliareinhart2
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1


Chemistry, 22.06.2019 13:00, carlinryan
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2
You know the right answer?
The rate constants of some reactions double with every 10 degree rise in temperature. Assume that a...
Questions in other subjects:




Health, 09.12.2020 14:00

Mathematics, 09.12.2020 14:00

Mathematics, 09.12.2020 14:00

English, 09.12.2020 14:00

Mathematics, 09.12.2020 14:00

Mathematics, 09.12.2020 14:00

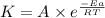
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0524/9978/6d953.png)
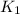 = rate constant at 271 K
= rate constant at 271 K = rate constant at 281 K =
= rate constant at 281 K = 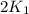
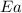 = activation energy for the reaction = ?
= activation energy for the reaction = ?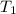 = initial temperature = 271 K
= initial temperature = 271 K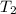 = final temperature = 281 K
= final temperature = 281 K![\log (\frac{2K_1}{K_1})=\frac{Ea}{2.303\times 8.314J/mole.K}[\frac{1}{271K}-\frac{1}{281K}]](/tpl/images/0524/9978/ceafb.png)