
Chemistry, 26.02.2020 16:59 valtrump3256
7) For the reaction 9A (g) + B (g) 5C(g) + 1/6 D (g), it takes 4 and a half minutes for the concentration of C to increase to 1.33 M. What would be the decrease in A during this time interval? a. 0.27 b. 0.044 c. 0.53

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, tifftifftiff5069
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1


Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
You know the right answer?
7) For the reaction 9A (g) + B (g) 5C(g) + 1/6 D (g), it takes 4 and a half minutes for the concen...
Questions in other subjects:


Mathematics, 22.10.2020 09:01

Physics, 22.10.2020 09:01

Physics, 22.10.2020 09:01


English, 22.10.2020 09:01



English, 22.10.2020 09:01

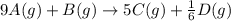
![\text{Rate of disappearance of }A=-\frac{1}{9}\times \frac{\Delta [A]}{\Delta t}](/tpl/images/0524/9437/d9f99.png)
![\text{Rate of formation of }C=+\frac{1}{5}\times \frac{\Delta [C]}{\Delta t}](/tpl/images/0524/9437/0b475.png)
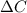 = change in concentration of C = 1.33 M
= change in concentration of C = 1.33 M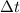 = change in time = 4.5 min
= change in time = 4.5 min![\frac{1}{9}\times \frac{\Delta [A]}{\Delta t}=\frac{1}{5}\times \frac{\Delta [C]}{\Delta t}](/tpl/images/0524/9437/4b36e.png)
![\frac{\Delta [A]}{\Delta t}=\frac{9}{5}\times \frac{\Delta [C]}{\Delta t}](/tpl/images/0524/9437/d1e06.png)
![\frac{\Delta [A]}{\Delta t}=\frac{9}{5}\times \frac{1.33M}{4.5min}](/tpl/images/0524/9437/041b7.png)
![\frac{\Delta [A]}{\Delta t}=0.53M/min](/tpl/images/0524/9437/813ba.png)


