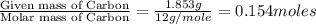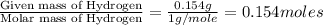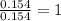Chemistry, 26.02.2020 16:40 kayla942783
A 2.007 g sample of a hydrocarbon is combusted to give 1.389 g of H 2O and 6.785 g of CO 2. What is the empirical formula of the compound? a. CH2 b. C2Hw c. C3H4 d. CH e. C5H12

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, dustinsampsin2486
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 17:30, rollercoasterbuddies
Why is the melting of ice a physical change ?
Answers: 1
You know the right answer?
A 2.007 g sample of a hydrocarbon is combusted to give 1.389 g of H 2O and 6.785 g of CO 2. What is...
Questions in other subjects:

Mathematics, 08.06.2021 22:30

English, 08.06.2021 22:30


English, 08.06.2021 22:30



Social Studies, 08.06.2021 22:30

Mathematics, 08.06.2021 22:30


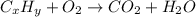
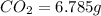
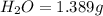
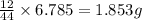 of carbon will be contained.
of carbon will be contained.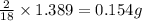 of hydrogen will be contained.
of hydrogen will be contained.