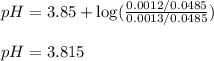Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:50, briansalazar17
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 17:30, katherineweightman
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1
You know the right answer?
A student performs a titration of 25.0 mL of 0.100 M lactic acid (HC3H5O3), using 0.050 M sodium hyd...
Questions in other subjects:

Mathematics, 09.02.2021 23:30

Computers and Technology, 09.02.2021 23:30

English, 09.02.2021 23:30

Mathematics, 09.02.2021 23:30

Biology, 09.02.2021 23:30


Chemistry, 09.02.2021 23:30

English, 09.02.2021 23:30

Engineering, 09.02.2021 23:30

History, 09.02.2021 23:30

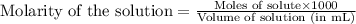
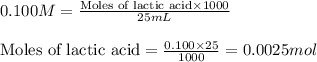
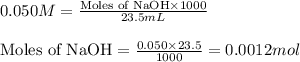
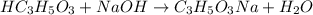
![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0524/8883/e4eea.png)
![pH=pK_a+\log(\frac{[C_3H_5O_3Na]}{[HC_3H_5O_3]})](/tpl/images/0524/8883/28764.png)
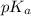 = negative logarithm of acid dissociation constant of lactic acid = 3.85
= negative logarithm of acid dissociation constant of lactic acid = 3.85![[HC_3H_5O_3]=\frac{0.0013}{0.0485}](/tpl/images/0524/8883/28573.png)
![[C_3H_5O_3Na]=\frac{0.0012}{0.0485}](/tpl/images/0524/8883/f1a5a.png)