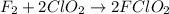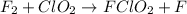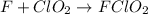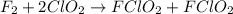Chemistry, 26.02.2020 04:19 kaylabay1997
A mechanism for the gas phase reaction of fluorine with chlorine dioxide that is consistent with the observed rate law is:
step 1 slow: F2 + ClO2 > FClO2 + F
step 2 fast: F > ClO2 + FClO2
What is the equation for the overall reaction?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, bbrogle5154
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1


Chemistry, 23.06.2019 06:30, destineedeal1
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1

Chemistry, 23.06.2019 19:00, MikeWrice3615
How long does it take light from the nearest star other than the sun to reach earth? a) less than 1 second b) about 1 hour c) about 1 month d) about 4 years
Answers: 1
You know the right answer?
A mechanism for the gas phase reaction of fluorine with chlorine dioxide that is consistent with the...
Questions in other subjects:



Mathematics, 26.11.2019 15:31

Mathematics, 26.11.2019 15:31





Physics, 26.11.2019 15:31