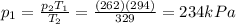On a summer day, you take a road trip through Death Valley, California, in an antique car. You start out at a temperature of 21°C, but the temperature in Death Valley will reach a peak of 56°C. The tires on your car hold 13.2 L of nitrogen gas at a starting pressure of 240 kPa. The tires will burst when the internal pressure (Pb) reaches 262 kPa. Answer the following questions and show your work.
• How many moles of nitrogen gas are in each tire?
• What will the tire pressure be at peak temperature in Death Valley?
• Will the tires burst in Death Valley? Explain.
• If you must let nitrogen gas out of the tire before you go, to what pressure must you reduce the tires before you start your trip? (Assume no significant change in tire volume.)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:30, trevorhenyan51
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 22.06.2019 23:00, Kykebailey2356
Which of two curves exhibits exponential growth
Answers: 1

Chemistry, 22.06.2019 23:30, hcllxxhhlpcj
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 02:00, hannabeth91
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1
You know the right answer?
On a summer day, you take a road trip through Death Valley, California, in an antique car. You start...
Questions in other subjects:

Mathematics, 14.01.2021 01:00

Mathematics, 14.01.2021 01:00

Mathematics, 14.01.2021 01:00

Biology, 14.01.2021 01:00





Mathematics, 14.01.2021 01:00

Arts, 14.01.2021 01:00

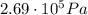
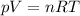
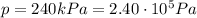
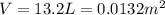
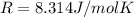
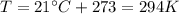
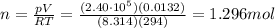
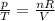
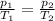
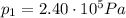 is the initial pressure
is the initial pressure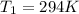 is the initial temperature
is the initial temperature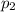 is the final pressure in Death Valley
is the final pressure in Death Valley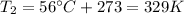 is the temperature in Death Valley
is the temperature in Death Valley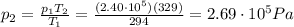
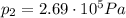
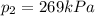
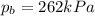
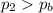
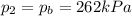
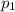 , the initial pressure at which the tires should be in order not to burst when the car arrives in the Death Valley.
, the initial pressure at which the tires should be in order not to burst when the car arrives in the Death Valley.