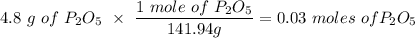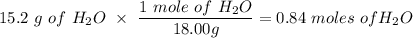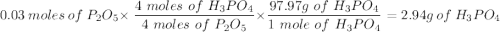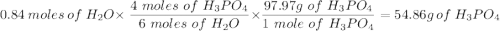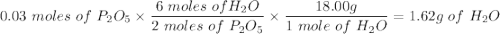Chemistry, 26.02.2020 02:54 preciousharrington13
Given this equation: 2P2O5 + 6H2O ---> 4H3PO4.
If you begin with 4.8 grams of P205 and 15.2 grams H2O, what will be your limiting reactant? How many grams of H3PO4 are produced?
Whenever I multiply P2O5 I always get 3.3 grams rather than 6.6 grams. However, when I multiply H2O I get the correct answer.
How many grams of the excess reagent remain unreacted?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, MrSavannahCat
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1


Chemistry, 22.06.2019 23:00, jolainjoseph01998
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
Given this equation: 2P2O5 + 6H2O ---> 4H3PO4.
If you begin with 4.8 grams of P205 and...
If you begin with 4.8 grams of P205 and...
Questions in other subjects:

Biology, 21.07.2019 14:30


History, 21.07.2019 14:30

Social Studies, 21.07.2019 14:30



Mathematics, 21.07.2019 14:30

Social Studies, 21.07.2019 14:30

Chemistry, 21.07.2019 14:30

Biology, 21.07.2019 14:30