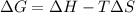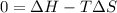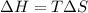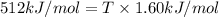As an approximation we can assume that proteins exist either in the native state and the denatured state. The standard molar enthalpy and entropy of the denaturation of a certain protein are 512 kj/mol and 1.60 kj/K mol. comment on the signs and magnitude of these quantities , and calculates the temperature at which the process favors the denatured state.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, rosieposie27
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1
You know the right answer?
As an approximation we can assume that proteins exist either in the native state and the denatured s...
Questions in other subjects:

Mathematics, 12.02.2021 21:50

Biology, 12.02.2021 21:50




Mathematics, 12.02.2021 21:50


English, 12.02.2021 21:50