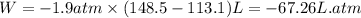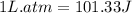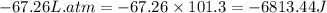Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 12:40, valenzueladomipay09u
How does concentration affect reaction rate
Answers: 2

Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
The combustion of 0.25 moles of octane gas (C8H18) to CO2 gas and H2O gas (vapor) against a constant...
Questions in other subjects:


History, 24.05.2021 16:40



Mathematics, 24.05.2021 16:40

Mathematics, 24.05.2021 16:40



Mathematics, 24.05.2021 16:40

Mathematics, 24.05.2021 16:40

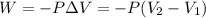
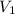 = initial volume = 113.1 L
= initial volume = 113.1 L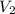 = final volume = 148.5 L
= final volume = 148.5 L