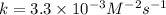Chemistry, 26.02.2020 00:55 02s54mcoupe
For the reaction A +B+ C D E, the initial reaction rate was measured for various initial concentrations of reactants. The following data were collected Trial A Bl Cl Initial rate 0.30 0.30 0.30 9.0x10 o 2.7x10-4 0.30 0.30 0.90 3.6x10-4 0.60 0.30 0.30 3.6x10-4 0.60 0.60 0.30
What is the value of the rate constant k for this reaction? When entering compound units, indicate multiplication of units explicitly using a multiplication dot (multiplication dot in the menu). For example, M−1⋅s−1. Express your answer to two significant figures and include the appropriate units. Indicate the multiplication of units explicitly either with a multiplication dot or a dash.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, fgcherubin
What happens to the atomic radius when an elctron is lost
Answers: 1


You know the right answer?
For the reaction A +B+ C D E, the initial reaction rate was measured for various initial concentrati...
Questions in other subjects:

Mathematics, 15.10.2021 04:10


Biology, 15.10.2021 04:20

Biology, 15.10.2021 04:20

Social Studies, 15.10.2021 04:20

History, 15.10.2021 04:20



Biology, 15.10.2021 04:20

 .
.![R=k[A]^a[B]^b[C]^c](/tpl/images/0524/1142/87958.png)
![[A]=0.30 M,[B]=0.30 M,[C]=0.30 M](/tpl/images/0524/1142/51008.png)
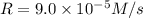
![9.0\times 10^{-5} M/s=k[0.30 M]^a[0.30 M]^b[0.30 M]^c](/tpl/images/0524/1142/34466.png) ...[1]
...[1]![[A]=0.30 M,[B]=0.30 M,[C]=0.90 M](/tpl/images/0524/1142/e516b.png)
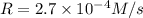
![2.7\times 10^{-4} M/s=k[0.30 M]^a[0.30 M]^b[0.90 M]^c](/tpl/images/0524/1142/a5767.png) ...[2]
...[2]![[A]=0.60 M,[B]=0.30 M,[C]=0.30 M](/tpl/images/0524/1142/90b57.png)
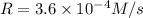
![3.6\times 10^{-4} M/s=k[0.60 M]^a[0.30 M]^b[0.30 M]^c](/tpl/images/0524/1142/97780.png) ...[3]
...[3]![[A]=0.60 M,[B]=0.60 M,[C]=0.30 M](/tpl/images/0524/1142/aaf09.png)
![3.6\times 10^{-4} M/s=k[0.60 M]^a[0.60 M]^b[0.30 M]^c](/tpl/images/0524/1142/d2528.png) ...[4]
...[4]![R=k[A]^2[B]^0[C]^1](/tpl/images/0524/1142/487c6.png)
![9.0\times 10^{-5} M/s=k[0.30 M]^2[0.30 M]^0[0.30 M]^1](/tpl/images/0524/1142/e707a.png)
![k=\frac{9.0\times 10^{-5} M/s}{[0.30 M]^2[0.30 M]^0[0.30 M]^1}](/tpl/images/0524/1142/db6d7.png)