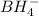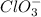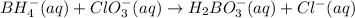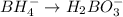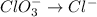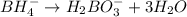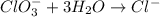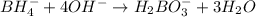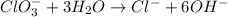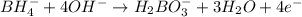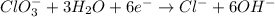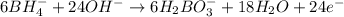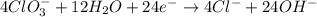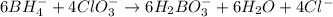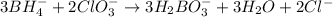Balance the following skeleton reaction and identify the oxidizing and reducing agents: Include the states of all reactants and products in your balanced equation. You do not need to include the states with the identities of the oxidizing and reducing agents.
BH4−(aq) + ClO3−(aq) → H2BO3−(aq) + Cl−(aq) [basic]
a. The oxidizing agent is:
b. The reducing agent is:

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adjjones2011
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 16:00, winnie45
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 16:50, briansalazar17
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
You know the right answer?
Balance the following skeleton reaction and identify the oxidizing and reducing agents: Include the...
Questions in other subjects:

Mathematics, 22.07.2020 20:01









Mathematics, 22.07.2020 20:01