
Hydrogen cyanide, HCN, is prepared from ammonia, air, and natural gas (CH4) by the following process: Hydrogen cyanide is used to prepare sodium cyanide, which is used in part to obtain gold from gold-containing rock. If a reaction vessel contains 5.90 g NH3, 11.0 g O2, and 4.67 g CH4, what is the maximum mass in grams of hydrogen cyanide that could be made, assuming the reaction goes to completion as written?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:02, aprilwilson3p8711m
How do you find a theoretical mass? is there a difference between theoretical mass and theoretical yield?
Answers: 1

Chemistry, 21.06.2019 18:30, ciarakelly636owuiup
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1

Chemistry, 21.06.2019 21:30, leslyrivera11
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 08:40, jaueuxsn
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2
You know the right answer?
Hydrogen cyanide, HCN, is prepared from ammonia, air, and natural gas (CH4) by the following process...
Questions in other subjects:


Physics, 24.03.2021 17:40




Mathematics, 24.03.2021 17:40


Mathematics, 24.03.2021 17:40

Biology, 24.03.2021 17:40

Mathematics, 24.03.2021 17:40

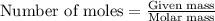 .....(1)
.....(1)
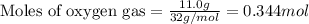
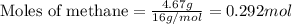


 of HCN
of HCN


