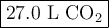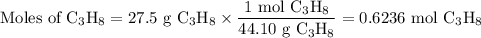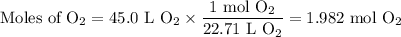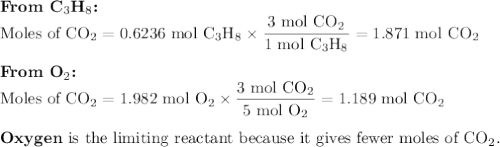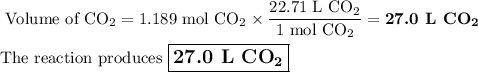The complete combustion of propane (C3H8) in the presence of oxygen yields CO2 and H2O:
C3H8 (...

Chemistry, 25.02.2020 22:19 tiaholmes31
The complete combustion of propane (C3H8) in the presence of oxygen yields CO2 and H2O:
C3H8 (g) + 5O2 (g) 3CO2 (g) + 4H2O (g)
a. Calcluate the volume of carbon dioixde ( at s. t.p.) that would be produced by the
combustion of 27.5 g of C3H8 burns in the presence of 45.0 L of O2.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:20, jordan2875
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 22.06.2019 23:10, carmenguabaoql9kv
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium. b)heavier than helium. c)the same weight as helium. d)dependent on the element that reacted with carbon.
Answers: 3

Chemistry, 23.06.2019 06:30, shateece
(04.05 hc) analyze the given diagram of the carbon cycle below. an image of carbon cycle is shown. the sun, a cloud, two trees, one on the left and the other on the right, an animal, lake, and a factory are shown in the image. an arrow is shown from the sun towards the left tree marked a. the sun is marked b. there is an arrow from the air above the clouds, marked c, towards the left tree. an arrow from a location close to the ground marked d points towards dead organisms, which is a label under the animal. an arrow marked e points from the right tree straight up to the clouds. an arrow marked f points from the animal straight up to the clouds. an arrow marked g points from the factory towards the air above the clouds, c. there is an arrow pointing from the air to the lake labeled carbonates in water, an arrow pointing down from dead organisms to fossils and fossil fuels, and an arrow from fossils to the factory. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer.
Answers: 2
You know the right answer?
Questions in other subjects:

Mathematics, 25.10.2021 04:50



Mathematics, 25.10.2021 04:50


Mathematics, 25.10.2021 04:50


English, 25.10.2021 04:50


Computers and Technology, 25.10.2021 04:50