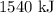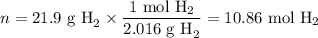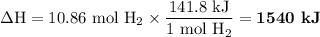Chemistry, 25.02.2020 22:09 lucyamine0
When 1 mole of hydrogen gas (H2) reacts with excess oxygen to form water at a constant pressure, 241.8 KJ of energy is released as heat. Calculate ΔH for a process in which 21.9 g sample of hydrogen gas (H2) reacts with excess oxygen at constant pressure.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, cbelew0001ouje4i
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 05:40, timmonskids6027
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1


Chemistry, 22.06.2019 12:20, missayers172
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3
You know the right answer?
When 1 mole of hydrogen gas (H2) reacts with excess oxygen to form water at a constant pressure, 241...
Questions in other subjects:

Chemistry, 11.04.2020 06:55



Physics, 11.04.2020 06:55



Chemistry, 11.04.2020 06:55


Biology, 11.04.2020 06:56

Physics, 11.04.2020 06:56